Stevioside (enzyme conversion method)
Steviol glycoside (enzymatic conversion method) is a natural zero-calorie sweetener rich in rebaudioside M, which is made from rebaudioside A derived from the leaves of Stevia by enzymatic catalysis.
Product characteristics
Good Taste: It is the best natural sweetener. It has no aftertaste or licorice taste and the taste is very close to sugar.
Low Calorie: Extremely low calorie, it’s about 1% of sucrose.
High Sweetness: The sweetness is 200-300 times that of sucrose.
Good Stability: Good stability against heat, acid and alkali, suitable for processing and cooking of various foods and beverages.
Suitable for people with high blood sugar: it’s low glycemic index and almost no calories are suitable for diabetics and other people who need to control blood sugar levels.
Applications
Beverages: Partially or completely replacing sucrose can improve the taste of beverages, reduce the bitterness of tea and coffee, and enhance the flavor of fruit drinks.
Ice Cream: Natural sweetness, soft texture, and increased flavor.
Fermented Milk: Effectively slows down the sour taste produced by yogurt fermentation, and the low calories will not cause blood sugar to rise and enhance the flavor.
Other Applications: It is widely used in betel nuts, candied fruits, pickles, roasted seeds and nuts, electronic cigarettes and other fields.
Promote the proliferation of Bifidobacterium and Lactobacillus in the human body, extend the shelf life, and prevent protein deterioration or browning, mold, etc. caused by rancidity.
Regulations
In 2013, the US FDA approved the safety of REB-M for the first time.
In 2015, Australia & New Zealand approved REB-M as a steviol glycoside component that can be used safely.
In 2019, EFSA approved REB-M as a food additive for use within the EU.
2020 Canadian Food Inspection Agency’s statement that REB-M is safe to use as a steviol glycoside component in food and beverages.
On March 13, 2024, the National Health Commission issued Document No. 2 of 2024, approving REB-M as a new food additive.
Used in accordance with GB2760-2024 Food Additive Usage Standards and relevant announcements from the National Health and Family Planning Commission.






 Rebaudioside M
Rebaudioside M  EN
EN English
English


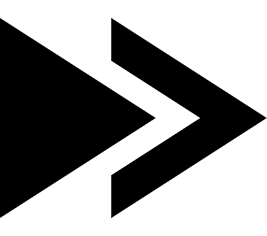 Rebaudioside M
Rebaudioside M
















.svg)












 Natural sweetener
Natural sweetener